ISSUE1614
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- F. Peter Swanson, M.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Susan Daron, Pharm D., Associate Editor: no disclosure or potential conflict of interest to report
- Discuss the efficacy and safety of casirivimab and imdevimab for treatment of COVID-19.
Revised 6/17/21: On May 21, 2021, the FDA revised the criteria by which an outpatient with COVID-19 can be deemed high-risk. On June 3, 2021, the FDA reissued its Emergency Use Authorization for casirivimab and imdevimab with new dosage and administration instructions. We have updated our article to reflect these changes.
Revised 1/6/2022: The Variants paragraph has been updated.
- Eligibility
- Mechanism of Action
- Clinical Study
- Variants
- Adverse Effects
- Dosage and Administration
- Availability
- Conclusion
- References
Table
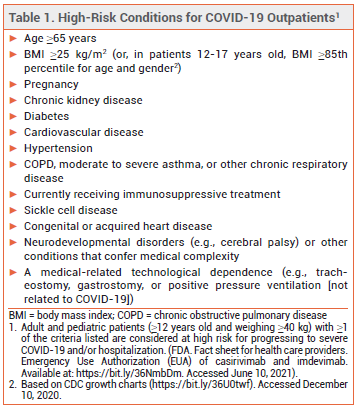
The FDA has issued an Emergency Use Authorization (EUA) for Regeneron's investigational monoclonal antibodies casirivimab and imdevimab (REGEN-COV) to be administered together by IV infusion or SC injection for treatment of mild to moderate COVID-19 in adults and pediatric patients (≥12 years old and weigh ≥40 kg) who are at high risk of progressing to severe COVID-19 and/or hospitalization.1
ELIGIBILITY – In May 2021, the FDA expanded the criteria by which a patient with COVID-19 can be considered at high risk for disease progression. All outpatients ≥12 years old who are overweight or pregnant or have cardiovascular disease, hypertension, or chronic respiratory disease are now eligible to receive monoclonal antibody treatment (see Table 1).2,3
Monoclonal antibodies may be associated with worse clinical outcomes when administered to hospitalized patients with COVID-19 who require high-flow oxygen or mechanical ventilation. Casirivimab and imdevimab are not authorized for use in patients who are hospitalized for COVID-19 or require oxygen therapy because of COVID-19.
MECHANISM OF ACTION — Casirivimab and imdevimab bind to different sites on the receptor binding domain of the spike protein of SARS-CoV-2, blocking its attachment to the human ACE2 receptor.
CLINICAL STUDY — Authorization of casirivimab and imdevimab was based on the interim phase 1/2 results of a double-blind trial (COV-2067) after 799 outpatients with mild to moderate COVID-19 had completed at least 28 days of study duration.3 The patients had been randomized to receive a single IV infusion of 2400 mg of casirivimab and imdevimab (1200 mg each), 8000 mg of casirivimab and imdevimab (4000 mg each), or placebo within 3 days of a positive SARS-CoV-2 test result. The time-weighted average reduction from baseline in viral load at day 7, the primary endpoint, was significantly larger with the pooled doses of the antibodies than with placebo. The predefined secondary endpoint of medically attended visits related to COVID-19 (def ned as hospitalization, emergency department visit, urgent care visit, or physician office/telemedicine visit) within 28 days occurred in 2.8% of patients who received casirivimab and imdevimab and in 6.5% of those who received placebo, a statistically significant difference. Results with the two doses of casirivimab and imdevimab were similar for all endpoints.
A post-hoc analysis found that 2% of casirivimab- and imdevimab-treated patients and 4% of placebo-treated patients were hospitalized or visited the emergency department for COVID-19 within 28 days after treatment; among patients at high risk for disease progression, the rates were 3% with casirivimab and imdevimab and 9% with placebo. The median time to symptom improvement was 5 days with casirivimab and imdevimab and 6 days with placebo.
In a subsequent phase 3 component of COV-2067, 3867 patients with mild to moderate COVID-19 and at least one risk factor for progression to severe disease were randomized to receive a single IV infusion containing 1200 mg of casirivimab and imdevimab (600 mg each), 2400 mg of casirivimab and imdevimab (1200 mg each), or placebo. The primary endpoint, COVID-19-related hospitalization or death from any cause by day 29, occurred in 1.0% of patients who received the 1200-mg dose compared to 3.2% of those concurrently randomized to placebo (HR 0.30; NNT 44.3), and in 1.3% of patients who received the 2400-mg dose compared to 4.6% of those concurrently randomized to placebo (HR 0.29; NNT 30.3). Based on these data, the FDA changed the authorized dose of casirivimab and imdevimab from 1200 mg to 600 mg of each antibody.3,4
Viral load reductions with IV and SC casirivimab and imdevimab appear to be similar, but clinical outcomes data with SC administration of the antibodies are limited.3 No studies directly comparing casirivimab and imdevimab with other monoclonal antibodies for COVID-19 are available.
VARIANTS — Casirivimab plus imdevimab is not active against the Omicron variant of SARS-CoV-2. The combination retains activity against the Delta variant of the virus.3
ADVERSE EFFECTS — Infusion- and injection-related reactions and anaphylaxis have been reported with use of casirivimab and imdevimab.3
DOSAGE AND ADMINISTRATION — Casirivimab and imdevimab are packaged both separately in 120 mg/mL vials and as a co-formulated solution in 60 mg/60 mg/mL vials. The vials must be refrigerated during storage and should be allowed to sit at room temperature for 20 minutes before dose preparation. The two antibodies are only authorized for administration together; they should be given as soon as possible after a positive SARS-CoV-2 test result and within 10 days of COVID-19 symptom onset.
The preferred authorized dosage of REGEN-COV is 600 mg of casirivimab and 600 mg of imdevimab administered as a single IV infusion after dilution in 50, 100, 150, or 250 mL of normal saline at a maximum rate of 310 mL/hr (180 mL/hr if diluted in 50 mL). If the solution cannot be used immediately after dilution, it can be refrigerated for up to 36 hours or left at room temperature for up to 4 hours, including infusion time. If the solution is refrigerated after dilution, it should sit at room temperature for 30 minutes before administration.
If IV infusion is not feasible and would lead to a delay in treatment, casirivimab and imdevimab can be administered subcutaneously. The authorized dosage is 600 mg of casirivimab and 600 mg of imdevimab administered as four consecutive 2.5-mL injections (2 injections of each antibody packaged separately, or 4 injections of the co-formulated formulation) into different sites on the thigh, the back of the upper arm, or the abdomen (except 2 inches around the navel). If a prepared syringe cannot be used immediately after dilution, it can be refrigerated or left at room temperature for up to 4 hours. If refrigerated, the syringe should sit at room temperature for 20 minutes before administration.
Casirivimab and imdevimab should be administered in a facility staffed and equipped to manage anaphylaxis. Patients should be monitored for hypersensitivity reactions during and for at least 1 hour after administration of the antibodies.
AVAILABILITY — Casirivimab and imdevimab will be allocated to state health departments by the US Department of Health and Human Services (HHS) based on case counts and severity of outbreaks. These state health departments will be responsible for allocating the antibodies to local health facilities.
CONCLUSION — The FDA has issued an Emergency Use Authorization (EUA) for the monoclonal antibodies casirivimab and imdevimab (REGEN-COV) to be administered together for treatment of COVID-19. Administration of the antibodies to high-risk outpatients recently diagnosed with mild to moderate COVID-19 reduced viral load and decreased the risk of hospitalization and emergency department visits. How casirivimab and imdevimab compare with bamlanivimab, another monoclonal antibody that is also available through an FDA EUA, remains to be determined.
- FDA News Release. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibodies for treatment of COVID-19. November 21, 2020. Available at: https://bit.ly/2IhDxP5. Accessed June 10, 2021.
- FDA News Release. Coronavirus (COVID-19) update: May 21, 2021. Available at: https://bit.ly/3fFoEUB. Accessed June 10, 2021.
- FDA. Fact sheet for health care providers. Emergency Use Authorization (EUA) of REGEN-COV™ (casirivimab and imdevimab). December 2021. Available at: https://bit.ly/3A4N0i7. Accessed January 6, 2022.
- FDA News Release. Coronavirus (COVID-19) update: June 4, 2021. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-june-4-2021. Accessed June 10, 2021.