ISSUE1644
Remdesivir (Veklury) in High-Risk Outpatients with COVID-19
The IV antiviral drug remdesivir (Veklury – Gilead) has been available for treatment of COVID-19 in hospitalized patients since 2020.1 Now, the FDA has approved remdesivir for treatment of mild to moderate COVID-19 in outpatients ≥12 years old who weigh ≥40 kg and are at high risk for progression to severe disease, including hospitalization or death; they also issued an Emergency Use Authorization (EUA) allowing its use in any other high-risk outpatient who weighs ≥3.5 kg.2,3
CLINICAL STUDIES — FDA approval of remdesivir for use in outpatients was based on the results of a double-blind trial in 562 nonhospitalized, SARS-CoV-2-positive adults who had developed symptoms of COVID-19 ≤7 days previously and had one or more risk factors for progression to severe COVID-19 (e.g., age ≥60 years, obesity, hypertension, diabetes). Patients were randomized to receive 3 days of IV treatment with remdesivir (200 mg on day 1 and 100 mg on days 2 and 3) or placebo. Hospitalization related to COVID-19 by day 28 occurred significantly less often with the active drug (0.7% vs 5.3% with placebo; HR 0.13 [95% CI 0.03-0.59]; NNT 21.8). No deaths occurred by day 28 in either group.4
No clinical trial data are available on use of remdesivir in patients aged <12 years or weighing <40 kg. Authorization of the drug for use in such patients was based on extrapolation of clinical and pharmacokinetic data from studies in adults.5
DOSAGE AND ADMINISTRATION — The recommended dosage of remdesivir in high-risk outpatients weighing ≥40 kg is 200 mg IV on day 1 and 100 mg IV on days 2 and 3. For patients weighing <40 kg, the recommended dose is 5 mg/kg on day 1 and 2.5 mg/kg on days 2 and 3. The drug should be infused over 30-120 minutes. Treatment should be started within 7 days of symptom onset. Patients receiving remdesivir should be monitored for hypersensitivity reactions during administration of the drug and for 1 hour after completion of each infusion. Remdesivir is not recommended for use in patients with an eGFR <30 mL/min (or, in full-term neonates 7-28 days old, a serum creatinine level ≥1 mg/dL).5
RECOMMENDATIONS — The NIH recommends a 3-day course of IV remdesivir as a third-line option for treatment of mild to moderate COVID-19 in high-risk outpatients ≥12 years old who weigh ≥40 kg; it should be used if Paxlovid (nirmatrelvir/ritonavir) and sotrovimab are inappropriate or unavailable. Remdesivir is the only drug that is currently authorized by the FDA for treatment of COVID-19 in persons aged <12 years or weighing <40 kg.6,7
- Remdesivir (Veklury) for COVID-19. Med Lett Drugs Ther 2020; 62:186.
- FDA News Release. FDA takes actions to expand use of treatment for outpatients with mild-to-moderate COVID-19. January 21, 2022. Available at: https://bit.ly/3AwPkj9. Accessed January 28, 2022.
- CDC. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare providers. October 14, 2021. Available at: https://bit.ly/3tWR8Rg. Accessed January 28, 2022.
- RJ Gottlieb et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 2022; 386:305.
- FDA. Fact sheet for health care providers. Emergency Use Authorization (EUA) of Veklury (remdesivir) for the treatment of coronavirus disease 2019 (COVID-19) in pediatric patients. January 2022. Available at: https://bit.ly/35ok8aw. Accessed January 28, 2022.
- NIH. The COVID-19 Treatment Guidelines Panel’s statement on therapies for high-risk, nonhospitalized patients with mild to moderate COVID-19. January 19, 2022. Available at: https://bit.ly/3fYk4jC. Accessed January 28, 2022.
- COVID-19 updates: NIH outpatient treatment guidelines. Med Lett Drugs Ther 2022; 64:32.
NIH Outpatient Treatment Guidelines
NIH guidelines1 now recommend that high-risk outpatients with mild to moderate COVID-19 who are ≥12 years old and weigh ≥40 kg receive antiviral treatment with (in order of preference) a 5-day course of oral nirmatrelvir with ritonavir (Paxlovid),2 a single IV infusion of the monoclonal antibody sotrovimab,3 a 3-day course of IV remdesivir (Veklury),4 or (in adults) a 5-day course of oral molnupiravir.5 Nirmatrelvir/ritonavir and sotrovimab are preferred to remdesivir mainly because of logistical concerns associated with IV infusion of remdesivir on 3 consecutive days. Molnupiravir should only be used when Paxlovid, sotrovimab, and remdesivir are inappropriate or unavailable because it is less effective than these preferred alternatives. The monoclonal antibody combinations casirivimab plus imdevimab (REGEN-COV) and bamlanivimab plus etesevimab are not currently authorized for use in the US because they lack activity against the Omicron variant of SARS-CoV-2.6
- Remdesivir (Veklury) for COVID-19. Med Lett Drugs Ther 2020; 62:186.
- Paxlovid for treatment of COVID-19. Med Lett Drugs Ther 2022; 64:9.
- An EUA for sotrovimab for treatment of COVID-19. Med Lett Drugs Ther 2021; 63:97.
- COVID-19 updates: Remdesivir (Veklury) in high-risk outpatients with COVID-19. Med Lett Drugs Ther 2022; 64:31.
- Molnupiravir for treatment of COVID-19. Med Lett Drugs Ther 2022; 64:10.
- FDA Statement. Coronavirus (COVID-19) update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the omicron variant. January 24, 2022. Available at: https://bit.ly/3GVGsWR. Accessed January 28, 2022.
Moderna COVID-19 Vaccine (Spikevax) Gains Full Licensure
The FDA has licensed the mRNA-based COVID-19 vaccine manufactured by Moderna (Spikevax) for use as a 2-dose primary series to prevent COVID-19 in adults.1,2 It is the second COVID-19 vaccine to receive full licensure in the US; the mRNA-based vaccine manufactured by Pfizer/BioNTech (Comirnaty) was licensed in 2021.
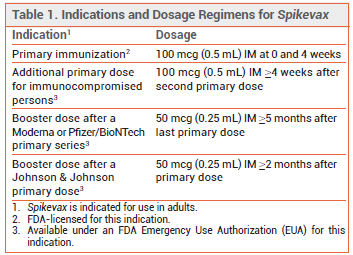
Spikevax remains available under an FDA Emergency Use Authorization (EUA) for use as a 3-dose primary series in immunocompromised adults and for booster immunization.3 A summary of indications for Spikevax can be found in Table 1.
- FDA News Release. Coronavirus (COVID-19) update: FDA takes key action by approving second COVID-19 vaccine. January 31, 2022. Available at: https://bit.ly/3L3RlIq. Accessed February 3, 2022.
- FDA authorizes Moderna COVID-19 vaccine. Med Lett Drugs Ther 2021; 63:9.
- FDA. Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). January 31, 2022. Available at: https://bit.ly/3nosylA. Accessed February 3, 2022.
Additional Content Available Online: COVID-19 Tables/Charts
See the latest information on COVID-19, including our continuously updated tables/charts on treatments, vaccines, and dosing recommendations.