ISSUE1679
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Discuss the efficacy of dapagliflozin (Farxiga) in patients with heart failure and any left ventricular ejection fraction.
The sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga – AstraZeneca) was approved by the FDA in 2020 to reduce the risk of cardiovascular death and hospitalization for heart failure (HF) in adults with heart failure with reduced ejection fraction (HFrEF).1 The indication has now been expanded to include a reduction in the risk of urgent HF visits and use in adults with any left ventricular ejection fraction (LVEF).
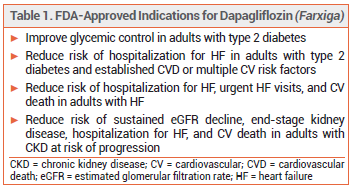
SGLT2 INHIBITORS AND HF — All currently available SGLT2 inhibitors have been shown to reduce the risk of hospitalization for HF by ~30% in patients with type 2 diabetes. Dapagliflozin is the second SGLT2 inhibitor to be approved for use in adults with any LVEF; empagliflozin (Jardiance) was the first.2 Current guidelines recommend dapagliflozin or empagliflozin for all patients with NYHA class II–IV HFrEF and for all those with HF with a mildly reduced or preserved ejection fraction (HFpEF).3,4 Canagliflozin (Invokana) and ertugliflozin (Steglatro) do not have a HF indication and are not recommended for treatment of HF. Sotagliflozin (Inpefa), a dual SGLT1 and 2 inhibitor, was recently approved by the FDA to reduce the risk of cardiovascular death, urgent HF visits, and hospitalization for HF in adults with either HF or type 2 diabetes, chronic kidney disease, and other cardiovascular risk factors; it will be reviewed in a future issue.
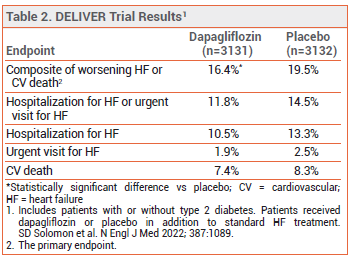
CLINICAL STUDIES ― FDA approval of the expanded indication was based on the results of a trial (DELIVER) in 6263 adults with chronic HF and an LVEF >40% who were randomized to receive dapagliflozin 10 mg or placebo once daily in addition to standard HF treatment (renin-angiotensin system inhibitor, sacubutril/valsartan, beta blocker, mineralocorticoid receptor antagonist, and/or diuretic). Over a median of 2.3 years, a composite of worsening HF or cardiovascular death, the primary endpoint, occurred in statistically significantly fewer patients in the dapagliflozin arm than in the placebo arm. The rates of hospitalization for HF, an urgent visit for HF, and cardiovascular death were each lower in the dapagliflozin arm (see Table 2). The results were similar in patients with an LVEF ≥60% or <60%, those with or without type 2 diabetes, and those with or without a recent hospitalization for HF.5
No trials comparing dapagliflozin with empagliflozin for the expanded indication are available.
DOSAGE AND COST ― For patients who require only glycemic control, the recommended starting dosage of dapagliflozin is 5 mg once daily; the dose can be increased to 10 mg if needed. The recommended dosage for all other indications is 10 mg once daily. Dapagliflozin should not be started in patients with an eGFR <25 mL/min/m2. The wholesale acquisition cost of 30-day supply of Farxiga is $565.30.6
CONCLUSION ― Addition of the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) to standard heart failure treatment reduces the risk of cardiovascular death and hospitalization for heart failure in patients with heart failure with any ejection fraction. Although no head-to-head trials are available, dapagliflozin appears to be similar in efficacy to the SGLT2 inhibitor empagliflozin (Jardiance), which is also approved for use in patients with heart failure with any ejection fraction.
- Dapagliflozin (Farxiga) – a new indication for heart failure. Med Lett Drugs Ther 2020; 62:102.
- In brief: Expanded heart failure indication for empagliflozin (Jardiance). Med Lett Drugs Ther 2022; 64:57.
- PA Heidenreich et al. 2022 AHA/ACC/HFSA guideline for management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022; 145:e895. doi:10.1161/cir.0000000000001063
- MM Kittleson et al. 2023 ACC expert consensus decision pathway on management of heart failure with preserved ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2023; 81:1835. doi:10.1016/j.jacc.2023.03.393
- SD Solomon et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022; 387:1089. doi:10.1056/nejmoa2206286
- Approximate WAC. WAC = wholesaler acquisition cost or manufacturer's published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. June 5, 2023. Reprinted with permission by First Databank, Inc. All rights reserved. ©2023. www.fdbhealth.com/policies/drug-pricing-policy.