ISSUE1689
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Review the data supporting FDA approval of the SGLT2 inhibitor empagliflozin (Jardiance) for improving renal outcomes in adults with chronic kidney disease.
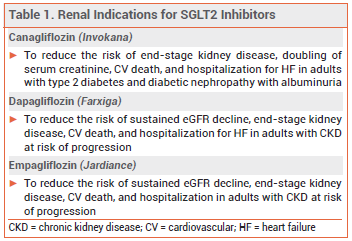
The sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (Jardiance – Boehringer Ingelheim/Lilly) is now FDA-approved to reduce the risk of sustained eGFR decline, end-stage kidney disease, cardiovascular death, and hospitalization in adults with chronic kidney disease (CKD) at risk of progression. It is also approved to improve glycemic control in patients ≥10 years old with type 2 diabetes, to reduce the risk of cardiovascular death and hospitalization for heart failure (HF) in adults with HF, and to reduce the risk of cardiovascular death in adults with type 2 diabetes and established cardiovascular disease.
OTHER SGLT2 INHIBITORS — The SGLT2 inhibitors canagliflozin (Invokana) and dapagliflozin (Farxiga) are also approved to improve renal outcomes (see Table 1). The SGLT2 inhibitors ertugliflozin (Steglatro) and bexagliflozin (Brenzavvy) and the SGLT1/2 inhibitor sotagliflozin (Inpefa) have not been approved by the FDA for any renal indications.1-3
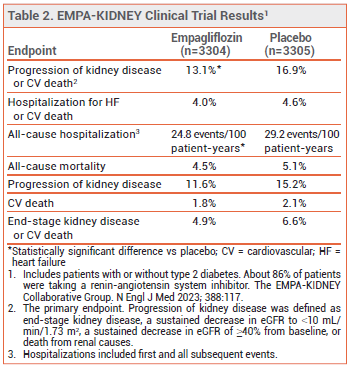
CLINICAL STUDIES — Approval of the new indication for empagliflozin was based on the results of a double-blind trial (EMPA-KIDNEY) in 6609 patients (with or without type 2 diabetes) with CKD (eGFR ≥20-<45 mL/min/1.73 m2, or eGFR ≥45-<90 mL/min/1.73 m2 with a urine albumin-to-creatinine ratio ≥200 mg/g). Patients were randomized to receive empagliflozin 10 mg or placebo once daily in addition to a renin-angiotensin system inhibitor. Over a median follow-up of 2 years, the incidence of the primary endpoint, a composite of progression of kidney disease or cardiovascular death, was statistically significantly lower with empagliflozin than with placebo. The rate of hospitalization for any cause was also statistically significantly lower with empagliflozin than with placebo, but the rates of all-cause mortality and a composite of hospitalization for heart failure or cardiovascular death were not (see Table 2).4
No trials directly comparing the renal benefits of empagliflozin with those of canagliflozin or dapagliflozin are available.
ADVERSE EFFECTS — SGLT2 inhibitors, including empagliflozin, can increase the risk of genital mycotic infection, urinary tract infection, volume depletion, hypotension, and ketoacidosis in patients with type 2 diabetes. In EMPA-KIDNEY, lower limb amputations occurred in 28 patients in the empagliflozin group and in 19 of those in the placebo group.
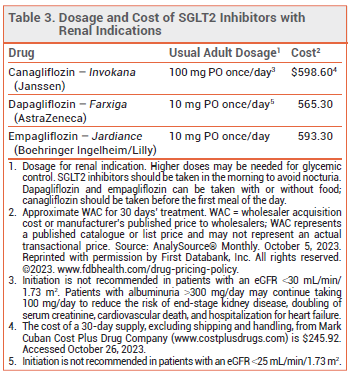
DOSAGE, ADMINISTRATION, AND COST — The recommended dosage of empagliflozin for all indications is 10 mg taken once daily in the morning. The dose can be increased to 25 mg in patients who need additional glycemic control.
- Drugs for type 2 diabetes. Med Lett Drugs Ther 2022; 64:177.
- Bexagliflozin (Brenzavvy) – a fifth SGLT2 inhibitor for type 2 diabetes. Med Lett Drugs Ther 2023; 65:130.
- Sotagliflozin (Inpefa) for heart failure. Med Lett Drugs Ther 2023; 65:114.
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023; 388:117. doi:10.1056/nejmoa2204233